herunterladen
2004 Microchip Technology Inc. DS00947A-page 1
AN947
INTRODUCTION
Powering today’s portable world poses many chal-
lenges for system designers. The use of batteries as a
prime power source is on the rise. As a result, a burden
has been placed on the system designer to create
sophisticated systems utilizing the battery’s full
potential.
Each application is unique, but one common theme
rings true: maximize battery capacity usage. This
theme directly relates to how energy is properly
restored to rechargeable batteries. While no single
method is ideal for all battery chemistries, an under-
standing of the charging characteristics of the battery,
along with the application’s requirements, is essential
when designing an appropriate and reliable battery-
charging system. Each method has its associated
advantages and disadvantages, with the particular
application (and its individual requirements)
determining the best method to use.
This application note focuses on the fundamentals of
charging Lithium-Ion/Lithium-Polymer batteries. In
particular, a linear, stand-alone solution utilizing
Microchip’s MCP73841 will be explored.
BATTERY OVERVIEW
A battery is a device that converts the chemical energy
contained in its active materials directly into electric
energy by means of an electrochemical oxidation-
reduction (redox) reaction. This type of reaction
involves the transfer of electrons from one material to
another through an electric circuit. In a non-electro-
chemical redox reaction, such as rusting or burning, the
transfer of electrons occurs directly and only heat is
involved.
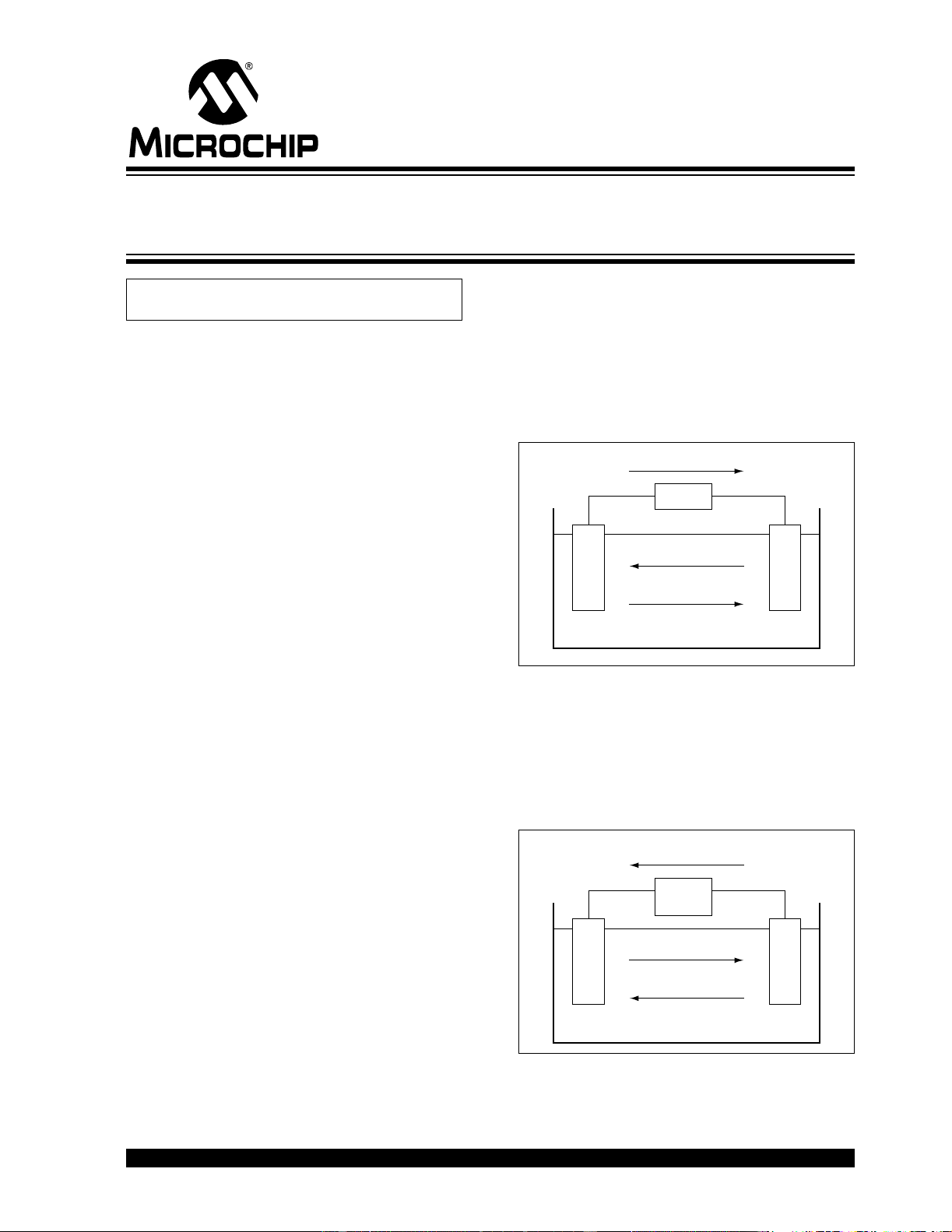
The operation of a battery during discharge is depicted
schematically in Figure 1. When the electrodes (posi-
tive and negative terminals of the battery) are con-
nected to an external load, electrons flow from the
anode, which is oxidized, through the external load to
the cathode. The cathode accepts the electrons and
the cathode material is reduced. The electric circuit is
completed in the electrolyte by the flow of anions
(negative ions) and cations (positive ions) to the anode
and cathode, respectively. By definition, the cathode
(oxidizing electrode) is the electrode that accepts
electrons from the external circuit and is reduced dur-
ing the electrochemical reaction. The anode (reducing
electrode) is the electrode which gives up electrons to
the external circuit and is oxidized during the electro-
chemical reaction. The electrolyte (ionic conductor)
provides the medium for transfer of charge, as ions,
inside the battery between the anode and cathode.
FIGURE 1: Discharge of a Battery.
When recharging a battery, the current flow is reversed,
with oxidation occurring at the positive electrode and
reduction at the negative electrode. As the anode is, by
definition, the electrode at which oxidation occurs and
the cathode where reduction occurs, the positive elec-
trode is now the anode and the negative electrode is
the cathode. Refer to Figure 2.
FIGURE 2: Charge of a Battery.
Author: Scott Dearborn
Microchip Technology Inc.
Anode
Cathode
Flow of Anions
Flow of Cations
Electrolyte
–
+
Load
Electron Flow
Anode
Cathode
Flow of Anions
Flow of Cations
Electrolyte
–
+
DC
Supply
Electron Flow
+
–
Power Management in Portable Applications: Charging
Lithium-Ion/Lithium-Polymer Batteries








