herunterladen
www.VadoseZoneJournal.org
| 780
2010, Vol. 9
Bromide Adsorp on by Reference
Minerals and Soils
Bromide, Br
−
, adsorp on behavior was inves gated on amorphous Al and Fe oxide, mont-
morillonite, kaolinite, and temperate and tropical soils. Bromide adsorp on decreased
with increasing solu on pH with minimal adsorp on occurring above pH 7. Bromide
adsorp on was higher for amorphous oxides than for clay minerals. Shi s in point of zero
charge (PZC) were observed on amorphous Al and Fe oxide following Br
−
adsorp on, sug-
ges ng an inner-sphere adsorp on mechanism for Br
−
on these surfaces. Ionic strength
eff ects indicated an inner-sphere adsorp on mechanism for Br
−
on kaolinite and an outer-
sphere adsorp on mechanism on amorphous Fe oxide. Two chemical surface complexa on
models, the constant capacitance model and the triple layer model, were able to describe
Br
−
adsorp on as a func on of solu on pH on all materials. For the oxides and clay minerals
and most of the soils the fi t of the constant capacitance model, containing an inner-sphere
adsorp on mechanism, was improved over that of the triple layer model, containing an
outer-sphere adsorp on mechanism, as measured by the overall variance, V
Y
. Bromide
adsorp on on amorphous Fe oxide as a func on of solu on pH and solu on ionic strength
was well described using the triple layer model. Our results indicate that Br
−
would most
likely not act as a conserva ve tracer at soil solu on pH values below 7. Therefore, we sug-
gest that researchers carefully evaluate the pH regime and mineralogy of their study site
before assuming that Br
−
can be applied as a conserva ve tracer for transport experiments.
Abbrevia ons: IOC, inorganic carbon; OC, organic carbon; PZC, point of zero charge.
A tracer is an identi able substance that can be followed through the course of a physical,
chemical, or biological process. An ideal hydrogeologic tracer is nontoxic, inexpensive, moves
with the water, is easy to detect in trace amounts, is chemically stable for a desired length of
time, is not present in large amounts in the water being studied, and is not sorbed by the solid
medium through which the water moves (Davis et al., 1980). In temperate regions Br
−
has
been considered to be an ideal tracer (Skaggs et al., 2002) that moves conservatively through
soils (Bowman, 1984; Jardine et al., 1988), with a retardation coe cient R = 1.
Nonconservative behavior (R ≠ 1) of Br
−
has sometimes been observed. e cause of tracer
movement that is faster than water ow (R < 1) has been attributed to anion exclusion from
conducting pores due to repulsion of Br
−
by negative charges on soil surfaces (Gerritse and
Adeney, 1992). Tracer movement that is slower than water ow (R > 1) has been attributed
to Br
−
adsorption on variably charged soil surfaces that have appreciable anion exchange
capacity (Boggs and Adams, 1992; Ishiguro et al., 1992; Seaman et al., 1995; Li et al., 1995;
Ritter et al., 2005; Wong and Wittwer, 2009). Methods of soil sampling and sample prepa-
ration of column experiments can signi cantly a ect Br
−
adsorption behavior (Boggs and
Adams, 1992). e Br
−
adsorbing surfaces in soils are considered to be Fe and Al oxides
and kaolinite (Boggs and Adams, 1992; Duwig et al., 1999; Ritter et al., 2005). Bromide
retardation on iron-coated sand columns was attributed to the ferrihydrite coating because
transport of Br
−
through uncoated quartz sand columns was conservative (Brooks et al.
(1998). Bromide retardation increased because anion exchange capacity increased with
decreasing pH below the PZC.
Very few studies of Br
−
adsorption have been conducted on soils (Li et al., 1995), clay
minerals (Weerasooriya and Wickramarathna, 1999), and oxides (Petkovic et al., 1994;
Chubar et al., 2005). Bromide adsorption was observed on the spodic B
h
horizon of
a spodosol but not on the surface A or the sandy E horizons. e Freundlich adsorp-
tion equation was t to the Br
−
adsorption isotherm data (Li et al., 1995). Freundlich
adsorption isotherm parameters were also obtained from Br
−
transport data in variably
charged sediments where tailing was observed (Korom, 2000). Bromide adsorption on
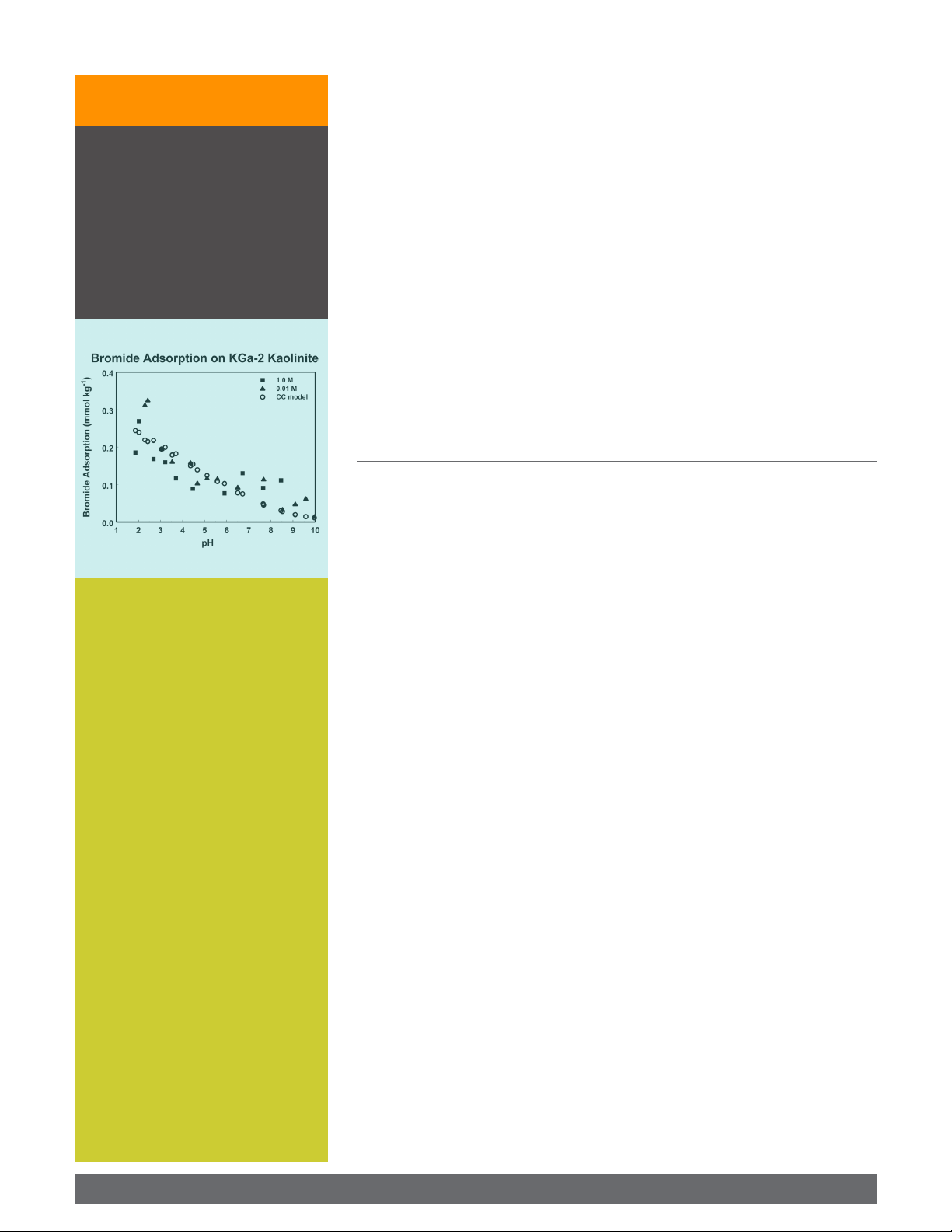
kaolinite decreased with increasing solution pH in the range 4 to 8. e magnitude of
Bromide adsorp on as a func on of
solu on pH and background electro-
lyte concentra on was determined
for a set of reference minerals and
soils. Adsorption was observed for
pH <7, indica ng that bromide would
not act as a conserva ve tracer for
such condi ons. Therefore cau on
is advised when using bromide as a
tracer under such condi ons.
S. Goldberg, USDA-ARS, U.S. Salinity Lab., 450
W. Big Springs Rd., Riverside, CA 92507. N.J.
Kabengi, Plant and Soil Sciences Dep., Univ. of
Kentucky, Lexington, KY 40546. *Correspon-
ding author (Sabine.Goldberg@ars.usda.gov).
Vadose Zone J. 9:780–786
doi:10.2136/vzj2010.0028
Received 16 Feb. 2010.
Published online 3 Aug. 2010.
© Soil Science Society of America
5585 Guilford Rd., Madison, WI 53711 USA.
All rights reserved. No part of this periodical may
be reproduced or transmi ed in any form or by any
means, electronic or mechanical, including photo-
copying, recording, or any informa on storage and
retrieval system, without permission in wri ng from
the publisher.
Original Research
Sabine Goldberg*
Nadine J. Kabengi








